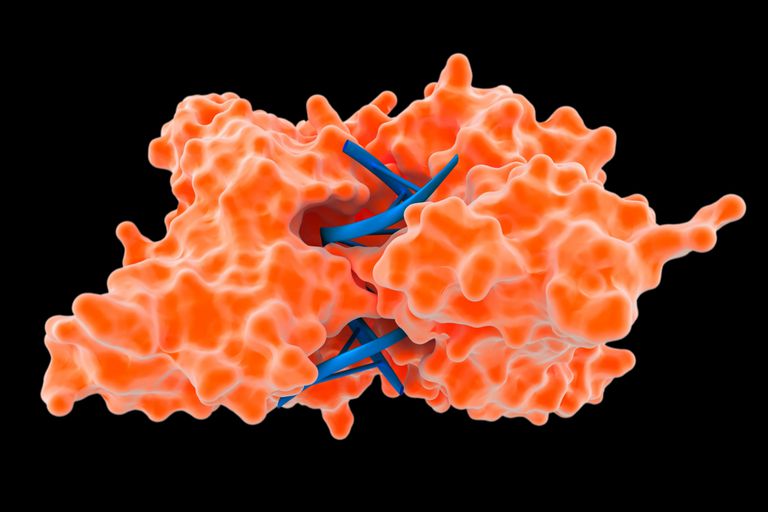
Restriction enzymes, also known as restriction endonucleases, are molecular scissors of the biological world. These enzymes are pivotal tools in molecular biology, enabling scientists to manipulate DNA with exquisite precision. But how exactly do they perform this seemingly magical feat of cutting DNA at specific locations? The answer lies in their unique recognition and cleavage mechanisms.
Imagine DNA as a long, intricate necklace composed of countless beads, each representing a nucleotide base (adenine, guanine, cytosine, and thymine). This necklace carries the genetic blueprint for an organism. Now, envision a tiny pair of scissors, the restriction enzyme, designed to cut this necklace only at a very specific sequence of beads. This specificity is the hallmark of restriction enzymes. They don’t just randomly chop up the DNA; instead, they recognize and bind to particular sequences, known as recognition sites.
Recognition Sites: The Enzyme’s Target
Each restriction enzyme possesses a unique affinity for a specific DNA sequence, typically 4 to 8 base pairs long. These sequences are often palindromic, meaning they read the same forwards and backward on opposite strands of the DNA double helix. For example, the restriction enzyme EcoRI, derived from *Escherichia coli*, recognizes the sequence GAATTC. Note how the complementary strand reads CTTAAG, which is the same sequence in the opposite direction.
This palindromic nature is crucial for the enzyme’s function. The enzyme molecule usually exists as a dimer, with two identical subunits. Each subunit can bind to one half of the palindromic sequence, effectively embracing the DNA at the recognition site. Think of it like two hands clasping onto a designated section of a rope.
The Binding Process: A Molecular Embrace
The binding of a restriction enzyme to its recognition site isn’t merely a physical attachment. It’s a precise molecular interaction governed by the principles of chemical complementarity. The enzyme’s active site, the region responsible for catalyzing the cutting reaction, perfectly complements the shape and chemical properties of the DNA sequence. Hydrogen bonds and van der Waals forces contribute to this tight, specific binding.
Initially, the enzyme might bind loosely to the DNA, scanning along the molecule until it encounters its cognate recognition sequence. Once found, the enzyme undergoes a conformational shift, tightening its grip on the DNA. This conformational change positions the enzyme’s catalytic machinery in close proximity to the phosphodiester bonds that link the nucleotides together. These bonds are the targets of the enzyme’s cutting action.
Cleavage Mechanisms: Blunt Ends Versus Sticky Ends
The cleavage mechanism is where the “cutting” actually happens. Restriction enzymes catalyze the hydrolysis of the phosphodiester bonds, effectively breaking the DNA backbone. However, the way in which they cut can vary, resulting in different types of DNA ends.
Some restriction enzymes, like *AluI*, cut both DNA strands at the same position within the recognition site, creating what are known as blunt ends. Imagine taking a pair of scissors and cutting a piece of paper straight across. The resulting ends are perfectly flush.
Other restriction enzymes, like *EcoRI*, cut each strand of the DNA at different positions, creating staggered cuts. This results in single-stranded overhangs, often referred to as “sticky ends” or “cohesive ends”. These overhangs are called “sticky” because they can easily base-pair with complementary sequences. Think of these as two puzzle pieces that perfectly fit together.
These sticky ends are incredibly useful in molecular cloning. Two DNA fragments cut with the same restriction enzyme will have compatible sticky ends, allowing them to anneal together. DNA ligase, another enzyme, can then seal the phosphodiester backbone, creating a continuous DNA molecule.
The Importance of Mg2+ Ions
Many restriction enzymes require magnesium ions (Mg2+) to function properly. These ions act as cofactors, assisting in the catalytic process. Mg2+ ions help to stabilize the transition state of the reaction, making it easier for the enzyme to cleave the phosphodiester bonds. They also play a role in positioning the water molecule that participates in the hydrolysis reaction.
Without Mg2+ ions, the enzyme might bind to the DNA but be unable to perform the cutting reaction. This provides a means of controlling the enzyme’s activity *in vitro* (in a test tube). By adding or removing Mg2+ ions, scientists can turn the enzyme’s activity on or off.
Applications of Restriction Enzymes
Restriction enzymes are indispensable tools in molecular biology, genetics, and biotechnology. They are used in a wide range of applications, including:
- DNA Cloning: Cutting and pasting DNA fragments into vectors for replication and expression.
- Gene Mapping: Determining the relative positions of genes on a chromosome.
- DNA Fingerprinting: Identifying individuals based on their unique DNA profiles.
- Genetic Engineering: Modifying the genetic makeup of organisms.
- Diagnostic Testing: Detecting the presence of specific DNA sequences in a sample.
In essence, restriction enzymes are the cornerstone of modern molecular biology. Their ability to precisely cut DNA at specific sequences has revolutionized our ability to manipulate and understand the genetic code.
Beyond the Basics: Star Activity
While restriction enzymes are highly specific under optimal conditions, they can sometimes exhibit a phenomenon known as “star activity.” This refers to a relaxation of their specificity, where they begin to cut DNA at sequences similar to, but not identical to, their canonical recognition sites.
Star activity can be induced by several factors, including high enzyme concentrations, non-optimal ionic strength, the presence of glycerol, or high pH. Under these conditions, the enzyme’s active site may become less stringent, allowing it to tolerate slight variations in the DNA sequence.
Scientists must be aware of star activity to avoid unintended cuts and ensure the accuracy of their experiments. Careful control of reaction conditions is crucial for maintaining the enzyme’s specificity.
Understanding how restriction enzymes function—from their initial binding to their precise cleavage mechanisms—is paramount for anyone working in the field of molecular biology. These enzymes are more than just molecular scissors; they are sophisticated molecular machines that have transformed our ability to manipulate and understand the very fabric of life.








Leave a Comment