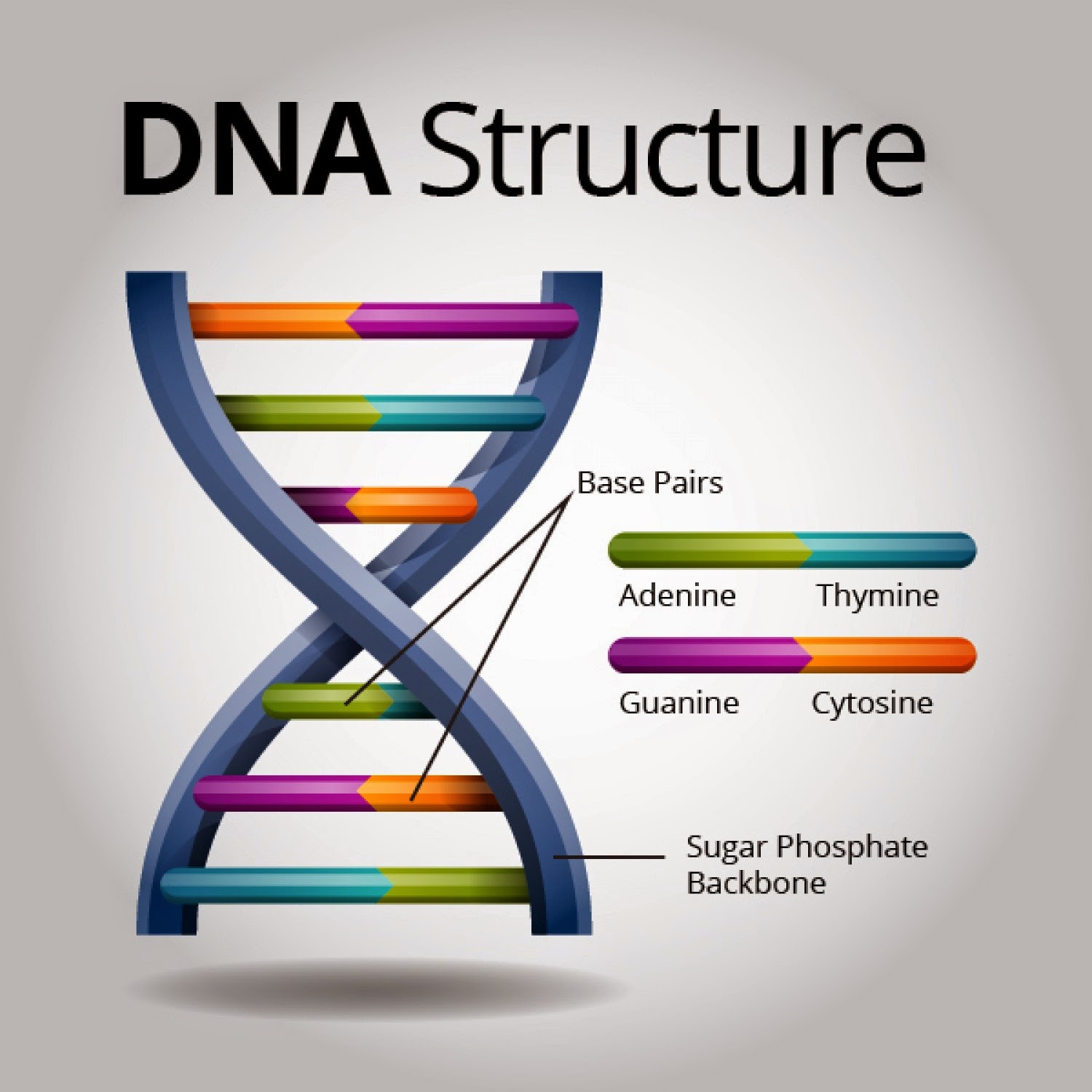
Deoxyribonucleic acid, or DNA, holds the blueprint of life. Understanding this blueprint requires the ability to decipher its sequence – the specific order of nucleotide bases (adenine, guanine, cytosine, and thymine) that constitute the genetic code. The process of determining this sequence is known as DNA sequencing, a cornerstone technique in modern biology and medicine. The fascination with DNA sequencing stems from its profound ability to unlock the secrets of heredity, disease, and evolution. Its applications span across various fields, from diagnosing genetic disorders to developing personalized medicine and tracking the spread of infectious agents.
Several methods have been developed for DNA sequencing over the years, each with its own strengths and limitations. These methods can be broadly categorized into first-generation sequencing (Sanger sequencing), second-generation sequencing (Next-Generation Sequencing or NGS), and third-generation sequencing (also known as Single-Molecule Sequencing). Each generation represents a significant leap in terms of throughput, cost, and speed.
Sanger Sequencing: The Gold Standard
Developed by Frederick Sanger in the 1970s, Sanger sequencing was the first widely adopted method for determining DNA sequences. It remained the workhorse of DNA sequencing for several decades, even being instrumental in the Human Genome Project. This method is based on the principle of chain termination during DNA replication.
How does it work? Sanger sequencing employs modified nucleotides called dideoxynucleotides (ddNTPs). These ddNTPs lack a 3′-OH group, which is essential for forming the phosphodiester bond that extends a DNA chain. When a ddNTP is incorporated into a growing DNA strand, it terminates further elongation. The reaction mixture contains a DNA polymerase, a DNA template to be sequenced, a primer to initiate DNA synthesis, normal deoxynucleotides (dNTPs), and a small amount of ddNTPs, each labeled with a fluorescent dye specific to a particular base (A, G, C, or T). As DNA polymerase synthesizes a new strand complementary to the template, it occasionally incorporates a ddNTP instead of a dNTP, resulting in chain termination at that specific base. This process generates a population of DNA fragments of varying lengths, each terminating with a fluorescently labeled ddNTP.
Following the reaction, the DNA fragments are separated by size using capillary electrophoresis. As the fragments migrate through the capillary, a laser excites the fluorescent dye on each fragment, and a detector records the emitted light. The color of the light indicates the identity of the terminal base, and the order in which the fragments elute from the capillary reveals the DNA sequence. Sanger sequencing is known for its high accuracy, typically exceeding 99.99%. However, it is relatively low-throughput and expensive compared to newer sequencing technologies.
Next-Generation Sequencing (NGS): Revolutionizing Genomics
Next-Generation Sequencing (NGS) technologies, also known as massively parallel sequencing, have revolutionized the field of genomics by enabling the rapid and cost-effective sequencing of millions or even billions of DNA fragments simultaneously. Several NGS platforms are available, each based on slightly different principles, but they all share a common workflow.
This workflow typically involves:
- Library Preparation: The DNA sample is fragmented, and adapters (short DNA sequences) are ligated to the ends of the fragments. These adapters serve as binding sites for primers used in subsequent amplification and sequencing steps.
- Clonal Amplification: The adapter-ligated DNA fragments are amplified, either in solution (e.g., emulsion PCR) or on a solid surface (e.g., bridge amplification). This clonal amplification generates clusters of identical DNA molecules, increasing the signal strength for sequencing.
- Sequencing: The amplified DNA fragments are sequenced using various methods, such as sequencing by synthesis (SBS) or sequencing by ligation (SBL). SBS involves incorporating fluorescently labeled nucleotides one at a time and detecting the emitted light as each nucleotide is added to the growing DNA strand. SBL, on the other hand, involves ligating short, fluorescently labeled oligonucleotide probes to the DNA fragments and detecting the emitted light as each probe is ligated.
- Data Analysis: The raw sequencing data is processed to generate sequence reads, which are then aligned to a reference genome or assembled de novo. Bioinformatics tools are used to identify variations, such as single nucleotide polymorphisms (SNPs), insertions, and deletions.
NGS platforms offer several advantages over Sanger sequencing, including much higher throughput, lower cost per base, and the ability to sequence complex mixtures of DNA fragments. However, NGS data can be more prone to errors, particularly in regions with repetitive sequences or low sequence complexity. Careful experimental design and data analysis are essential to ensure the accuracy and reliability of NGS results.
Third-Generation Sequencing: Long Reads and Single Molecules
Third-generation sequencing technologies, also known as single-molecule sequencing, offer the ability to sequence long DNA fragments (tens of thousands of base pairs) without the need for prior amplification. These technologies overcome some of the limitations of NGS, particularly in sequencing complex genomes with repetitive elements or structural variations.
Two prominent third-generation sequencing platforms are:
- Pacific Biosciences (PacBio) Sequencing: PacBio sequencing uses a technique called Single Molecule Real-Time (SMRT) sequencing. In SMRT sequencing, a DNA polymerase is attached to the bottom of a zero-mode waveguide (ZMW), a tiny well that allows only the light emitted from the polymerase active site to be detected. Fluorescently labeled nucleotides are added to the ZMW, and as the polymerase incorporates each nucleotide into the growing DNA strand, the fluorescent signal is detected. The duration and intensity of the signal provide information about the identity of the nucleotide.
- Oxford Nanopore Sequencing: Oxford Nanopore sequencing uses a different approach. A protein nanopore is embedded in an electrically resistant membrane. As a DNA molecule passes through the nanopore, it causes changes in the ionic current flowing through the pore. These changes in current are unique to each nucleotide base, allowing the DNA sequence to be determined.
Third-generation sequencing technologies offer several advantages, including long read lengths, the ability to detect DNA modifications (e.g., methylation), and the potential for real-time sequencing. However, they also have some limitations, such as lower accuracy compared to Sanger sequencing and NGS, and higher cost per base.
In conclusion, DNA sequencing is a powerful tool that has transformed our understanding of biology and medicine. From the pioneering work of Sanger sequencing to the revolutionary advances of NGS and third-generation sequencing, each method has contributed to our ability to decipher the genetic code and unlock the secrets of life. The ongoing development of new and improved sequencing technologies promises to further accelerate our understanding of genomics and its applications in various fields.








Leave a Comment